Abstract
Background
Circulating miRNAs have been shown to be useful diagnostic biomarkers of various diseases, including cancers, viral infections, nervous system disorders, and diabetes. Furthermore, measuring miRNA expression has the potential to predict the efficacy and/or adverse events of several chemotherapeutic agents. A few studies on multiple myeloma (MM) have evaluated miRNA in serum/plasma samples to determine the efficacy or toxicity of specific MM treatments.
We aimed to explore the association between the expression level of specific miRNAs in plasma and the efficacy or severity of bortezomib (Btz)-related toxicity by performing a comprehensive analysis of miRNAs from patients with newly diagnosed MM (NDMM) treated with Btz containing regimen.
Materials & Methods
Fifty-four plasma samples were analyzed from transplant-ineligible NDMM patients enrolled in a randomized phase II study comparing two less-intensive regimens of melphalan, prednisolone, and Btz (MPB) (JCOG1105; jRCTs031180097, Br J Haematol. 2021.). Prior to sample collection, patients provided informed consent to participate in the JCOG-BioBank Japan Biorepository project. Plasma samples, collected before the initiation of MPB therapy, were subjected to comprehensive miRNA expression analysis using a 3D Gene Human miRNA Oligo Chip. All miRNA expression levels were normalized to log2 scale using global normalization. We then examined whether the expression level of each miRNA was associated with the severity of Btz-induced toxicities, such as Btz-induced peripheral neuropathy (BiPN), skin disorders, pneumonitis, and response to MPB therapy. A multivariate permutation test with Welch's t-statistic was used for multiple comparisons of the miRNA expression levels between the two groups categorized according to their response to MPB therapy or the grade of toxicity. That is, non-complete response (CR) vs. CR, BiPN and skin disorders grade 0-1 vs. grade 2 or higher, and pneumonitis grade 0 vs. grade 1 or higher. Furthermore, we used LASSO regression analysis to assess the constructability of a prediction model for the risk of BiPN and skin disorders, as well as the response to MPB therapy.
Results and Discussion
In total, 2565 miRNAs were found in 54 samples. Among these, 1178 miRNAs with missing values in one or fewer samples were chosen. Then, we assessed the association between the expression levels of each miRNA and the toxicities or responses to MPB therapy. The expression levels of 67 and 26 miRNAs were associated with the severity of BiPN and skin disorders, respectively. Three and five miRNAs were linked to the onset of pneumonitis and the response to MPB therapy, respectively.
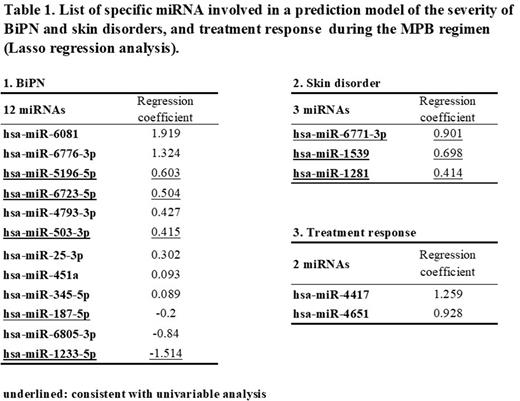
Following that, we used 1064 miRNAs expressed in all samples and attempted to build a predictive model using LASSO regression analysis. We adapted parameters to select optimal regularization parameters, and the minimum value of Akaike information criterion was applied. Table 1 shows that 12 and 3 miRNAs were identified as potential predictors of the severity of BiPN and skin disorders, respectively. In terms of the MPB therapy response, two miRNAs have been identified for predicting CR with MPB therapy.
Among the 12 miRNAs chosen as candidate predictive markers for BiPN severity, hsa-miR-451a has been shown to suppress the expression of multi-drug resistance 1 (MDR1), a Btz drug transporter. High expression levels of this circulating miRNA may be linked to repressing the expression of MDR1 in neurological cells, leading to Btz accumulation and worsening of BiPN during continuous Btz treatment.
Conclusions
Specific miRNAs can be associated with the severity of Btz-induced toxicity and the response to treatment in patients with MM. Circulating miRNA profiling may serve as a potential candidate biomarker for predicting Btz-induced toxicity and response to treatment in patients with MM. Our exploratory results need to be confirmed through further validation studies.
Disclosures
Ri:Celgene: Honoraria, Research Funding; Daiichi Sankyo: Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Janssen Pharmaceutical: Honoraria. Iida:Takeda Pharmaceutical: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Celgene: Honoraria, Research Funding; AbbVie: Research Funding; Amgen BioPharma: Research Funding; Pfizer: Consultancy, Research Funding; Ono Pharmaceutical: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Janssen Pharmaceutical: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Research Funding; Otsuka: Research Funding; Caelum: Research Funding. Maruyama:CHUGAI PHARMACEUTICAL: Honoraria, Research Funding; Kyowa Kirin: Honoraria, Research Funding. Fukuhara:Nippon Shinyaku: Honoraria; Abbvie: Consultancy; Kyowa kirin: Honoraria; Janssen: Honoraria; Eisai: Honoraria; Dainippon Sumitomo: Honoraria; Incyte: Research Funding; Genmab: Research Funding; BMS: Honoraria, Research Funding; Chugai pharma: Honoraria, Research Funding; Bayer: Research Funding; Novartis: Consultancy, Honoraria; HUYA: Consultancy; Eli Lilly: Consultancy; AstraZeneca: Consultancy, Honoraria; Ono pharma: Honoraria; Celgene: Honoraria, Research Funding; Sanofi: Honoraria; Symbio: Honoraria; Takeda: Honoraria. Miyazaki:Bristol-Myers Squibb: Honoraria; Symbio: Honoraria; Janssen Pharmaceutical: Honoraria; Nippon-Shinyaku: Honoraria; Celgene: Honoraria; Eisai: Honoraria; Chugai Pharmaceutical: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Zenyaku Kogyo: Research Funding; Kyowa Kirin: Honoraria, Research Funding; Meiji Seika: Honoraria; AbbVie: Honoraria; Novartis Pharma: Honoraria; Takeda Pharmaceutical: Honoraria. Tsujimura:Takeda: Honoraria; Chugai: Honoraria; Eisai: Honoraria; Janssen: Honoraria; Kyowa Kirin: Honoraria; Nippon Shinyaku: Honoraria. Yoshimitsu:CSL Behring: Honoraria; Novartis Japan: Honoraria; Otsuka Pharmaceutical: Honoraria; Sanofi: Honoraria; DAIICHI SANKYO COMPANY, LIMITED.: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria. Tsukasaki:Bristol Myers Squibb: Research Funding; HUYABIO: Consultancy, Research Funding; Solasia Pharma: Consultancy; Kyowa Kirin: Research Funding; Bayer Pharma: Research Funding; Daiichi Sankyo: Research Funding; Ono Pharmaceutical: Consultancy; Eisai: Honoraria; Yakuruto: Consultancy; Regeneron Pharmaceuticals Inc.: Research Funding; Chugai Pharmaceutical: Honoraria; Meiji Seika Pharma: Consultancy, Honoraria, Research Funding. Nagai:Eisai: Honoraria, Research Funding; Novartis: Honoraria; Janssen: Honoraria, Research Funding; Mudi Pharma: Honoraria, Research Funding; Eli Lilly and Company: Honoraria; AstraZeneca: Honoraria, Research Funding; Ono: Honoraria, Research Funding; Celgene: Honoraria; Sumitomo Pharma: Honoraria; Nippon Shinyaku: Honoraria, Research Funding; IQVIA: Research Funding; Labcorp: Research Funding; Parexel: Research Funding; Takeda: Honoraria, Research Funding; Chugai: Honoraria, Research Funding; Bristol Myers Squib: Honoraria; Kyowa Kirin: Honoraria, Research Funding; lncyte: Research Funding; Daiichi-Sankyo: Research Funding; Solasia: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal